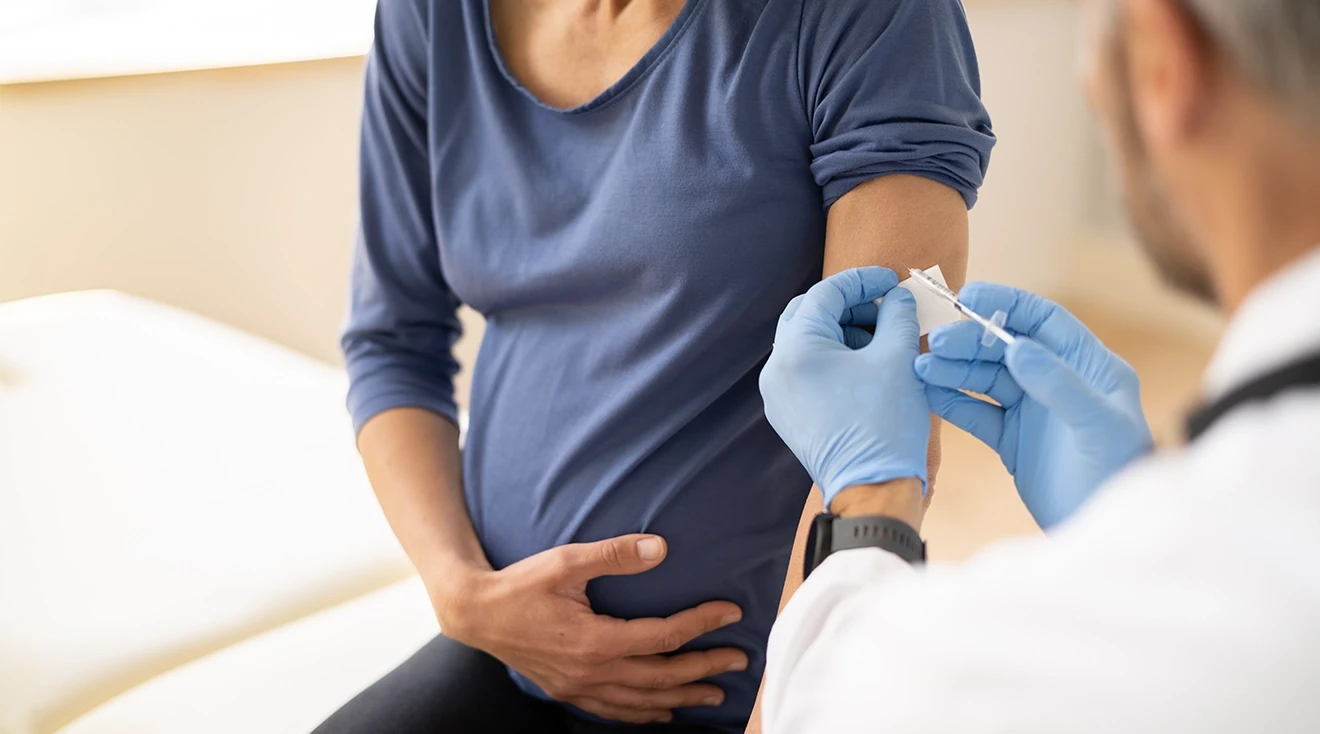
FDA Approves RSV Vaccine for Expectant Moms to Protect Newborns
Following a winter of record-high RSV cases and hospitals pushed to capacity, the first vaccine designed for pregnant moms to reduce severe RSV cases in newborns is now one step closer to rollout.
The Food and Drug Administration (FDA) announced its approval of Pfizer’s Abrysvo vaccine on August 21. To determine the safety and efficacy of the new vaccine, the organization reviewed information from an expert advisory panel and the results of three different randomized, double-blinded, placebo-controlled trials during which thousands of women received the vaccine.
The FDA has approved the vaccine for use between 32-36 weeks of pregnancy. The singular shot can be given during a regular prenatal visit by a licensed clinician. The organization encourages pregnant mothers to discuss the vaccine with their doctor to determine if it may or might not be right for them. Below we outline the two common questions that bear discussion with any new vaccine. Is it safe? Is it effective?
Is Pfizer’s RSV Vaccine effective?
The FDA has agreed that the vaccine is effective in preventing serious RSV cases in babies less than six months old. Pfizer’s RSV vaccine was tested in about 7,000 expectant mothers after the 24th week of pregnancy. About half received a placebo, and half were given the vaccine as a shot.
For the first 90 days after birth, only six infants in the vaccination group had a serious case of RSV, compared with 33 in the placebo group, translating to an efficacy of nearly 81.8 percent. In the first six months, during which baby is most likely to be hospitalized with a severe case of RSV, only 19 babies in the treatment group fell seriously ill compared to 62 in the placebo group.
RSV typically causes mild, cold-like symptoms among most adults and older children. Still, it can result in severe complications for babies younger than 12 months, as it spreads to the lower respiratory tract, causing pneumonia and bronchiolitis. Around 80,000 children each year are sent to the hospital with a severe case—making RSV the leading cause of hospitalization for kids under 1.
Encouragingly, even outside of RSV, trial results show the vaccine to be 57 percent effective at preventing lower respiratory tract infections that require doctor visits in baby’s first 90 days.
Is Pfizer’s RSV Vaccine safe?
After reviewing the data, the FDA has deemed the vaccine safe for pregnant women between 34-36 weeks. The most commonly reported side effects by pregnant individuals who received Abrysvo were pain at the injection site, headache, muscle pain and nausea.
Premature delivery was reported in 5.6 percent of the pregnancies in the treatment group, compared with 4.7 percent in the placebo group. This difference was deemed not statistically significant—meaning that the difference was not large enough to be attributed to the vaccine and could instead have been due to chance or other factors during the mother’s pregnancy. Still, due to this flag, the organization upped the vaccination timeline so the shot would be given no less than 34 weeks into a pregnancy.
The FDA is requiring the company to continue to conduct studies to assess the risk of preterm birth and to assess hypertensive disorders of pregnancy, including pre-eclampsia.
Other companies outside of Pfizer are also diligently working on RSV vaccinations. In mid-August, Sanofi and AstraZeneca’s Nirsevimab vaccine was approved by the FDA and recommended by the American Academy of Pediatrics (AAP). Unlike Pfizer’s vaccine, the Nirsevimab vaccine would be given directly to infants born in fall or winter, potentially quelling any worries around preterm births.
You can learn more about RSV and the other two respiratory illnesses that made up this winter’s “tripledemic” here.
Please note: The Bump and the materials and information it contains are not intended to, and do not constitute, medical or other health advice or diagnosis and should not be used as such. You should always consult with a qualified physician or health professional about your specific circumstances.
Navigate forward to interact with the calendar and select a date. Press the question mark key to get the keyboard shortcuts for changing dates.