Parents Can Give Kids Expired EpiPens as Shortage Continues, FDA Says
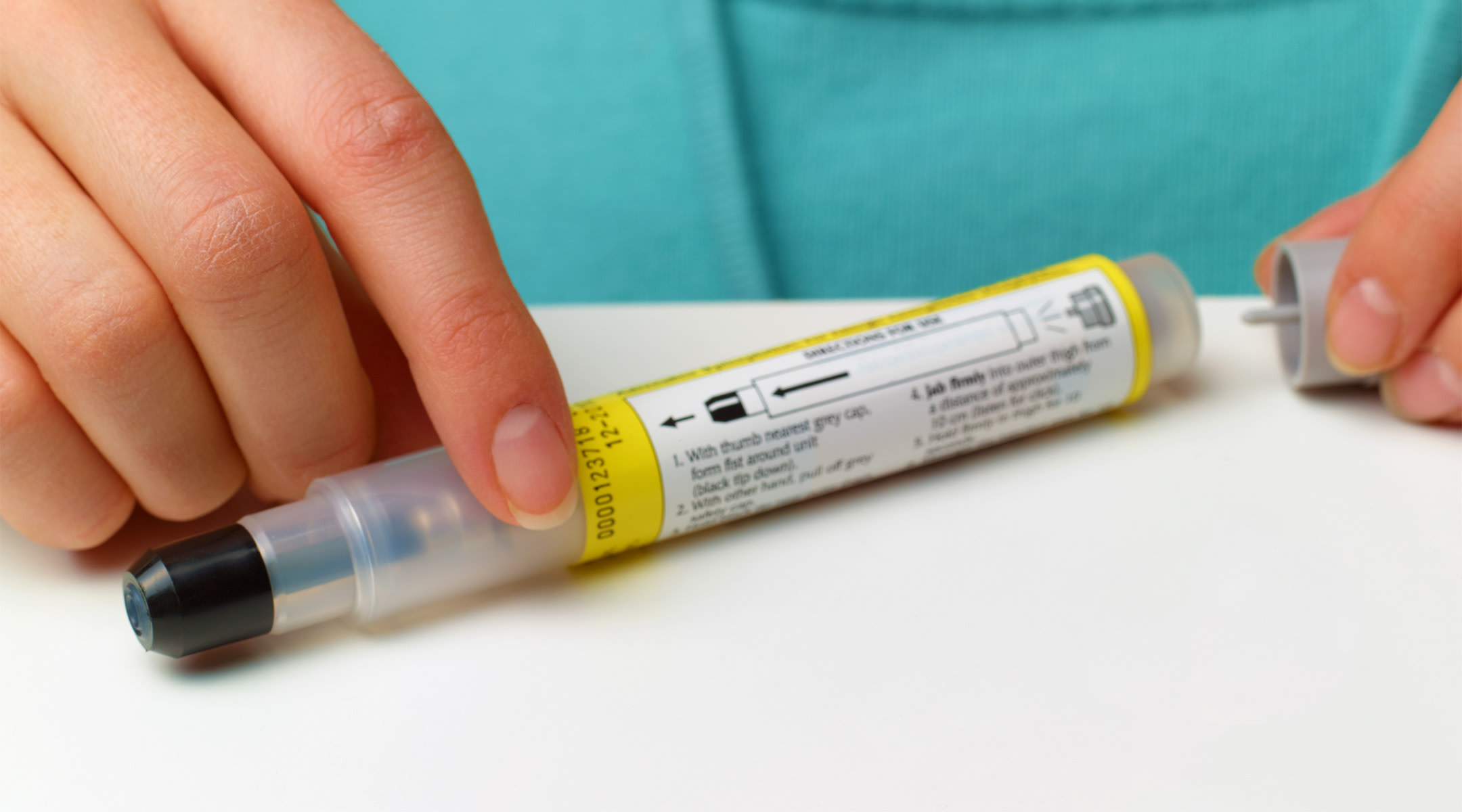
An ongoing shortage of EpiPens—those emergency injectors filled with an allergy-combatting dose of epinephrine—has left parents of children with severe allergies scrambling to outfit their school-bound kids with the lifesaving meds. If you haven’t managed to get your hands on any, you can now breathe a sigh of relief: The Food and Drug Administration (FDA) just okayed the use of certain expired EpiPens in case of emergencies.
Since spring, the availability of EpiPens has been limited in certain areas of the country due to supply disruptions and manufacturing issues, and parents are still struggling to find them.
Recognizing the urgent need, the FDA reviewed manufacturer data on the drugs’ safety beyond its advertised 20-month shelf-life and says certain EpiPens and its authorized generic version (made by Mylan) can be used beyond the labeled expiration date—as long as it’s no more than four months past expiration. It also depends on the batch and the amount of medicine in the EpiPen. A complete list of which EpiPens qualify for the extension are listed on the FDA’s website.
“Many patients rely on self-injectable epinephrine products, such as EpiPen, to reverse life-threatening reactions to bee stings or other allergens for either themselves or for their children. We are doing everything we can to help mitigate shortages of these products, especially ahead of the back-to-school season,” Janet Woodcock, MD, director of the FDA’s Center for Drug Evaluation and Research said in a recent statement. “We’re hopeful this action will ensure patients have access to this important medication and provide additional peace-of-mind to parents as the agency works with the manufacturer to increase supply.”
There is one additional caveat. If and when a replacement product becomes available during this temporary extension period, officials are recommending parents dispose of the expired EpiPens and replace them with new versions as soon as possible.
Just earlier this month, the FDA approved a generic version of EpiPen made by Israeli company Teva Pharmaceuticals, the first generic option available on the market that isn’t sold by Mylan. FDA Commissioner Scott Gottlieb called the move part of the agency’s “longstanding commitment to advance access to lower cost, safe and effective generic alternatives once patents and other exclusivities no longer prevent approval.” But exactly when this generic will be available in stores is still unclear.
If you need another option, CVS is offering an EpiPen alternative called Adrenaclick that costs $110 for a set of two, which is a fraction of EpiPen’s $600 price tag.
If you’re concerned about your child’s allergies, check out this emergency action plan from the American Academy of Pediatrics in addition to picking up your EpiPens.
Please note: The Bump and the materials and information it contains are not intended to, and do not constitute, medical or other health advice or diagnosis and should not be used as such. You should always consult with a qualified physician or health professional about your specific circumstances.
Navigate forward to interact with the calendar and select a date. Press the question mark key to get the keyboard shortcuts for changing dates.