FDA Approves COVID-19 Vaccines for Kids 6 Months and Up
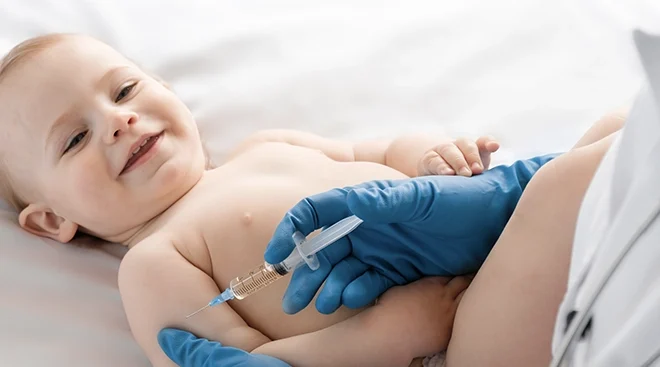
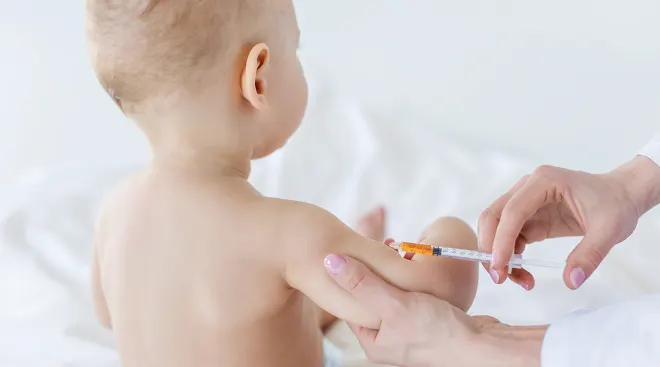
Today, the US Food and Drug Administration (FDA) authorized emergency use of the Moderna COVID-19 vaccine and the Pfizer-BioNTech COVID-19 vaccine for the prevention of COVID-19 in children as young as 6 months old. While FDA authorization is not the final step in the process (the Centers for Disease Control and Prevention (CDC) must also issue its recommendation), according to a statement by the White House, vaccines could be available by next week at pharmacies, doctor’s offices and hospitals.
“Many parents, caregivers and clinicians have been waiting for a vaccine for younger children, and this action will help protect those down to 6 months of age. As we have seen with older age groups, we expect that the vaccines for younger children will provide protection from the most severe outcomes of COVID-19, such as hospitalization and death,” said FDA Commissioner Robert M. Califf, MD, in a press release. “Those trusted with the care of children can have confidence in the safety and effectiveness of these COVID-19 vaccines and can be assured that the agency was thorough in its evaluation of the data.”
The FDA conducted ongoing, randomized, blinded, placebo-controlled clinical trials in the United States and internationally to determine the safety and effectiveness of both Moderna and Pfizer vaccines.
Over 2,800 children were involved in the study, ranging in age from 6 months to 5 years old. In both clinical trials, only mild side effects were observed, most commonly pain, redness and swelling at the injection site and fever. Pfizer and BioNTech reported last month that their Covid-19 vaccine was 80.3 percent effective in preventing Covid-19 cases that produced symptoms in children under 5 years old. In comparison, Moderna reported 43.7 percent efficacy for children between 6 months and 2 years.
You can read the full safety and effectiveness reports from the clinical trials here. “As with all vaccines for any population, when authorizing COVID-19 vaccines intended for pediatric age groups, the FDA ensures that our evaluation and analysis of the data is rigorous and thorough,” said Peter Marks, MD, director of the FDA’s Center for Biologics Evaluation and Research.
The president of the American Academy of Pediatrics, Moira Szilagyi, MD, had previously urged the FDA and CDC to review and authorize the vaccine for all age groups, saying, “Our youngest children deserve to have the same protection from a vaccine as every other group in our society.” Now parents can ensure that even the youngest members of their family are protected.
Learn more about COVID-19 in babies and toddlers, and visit the FDA’s COVID-19 vaccination page for more information about COVID-19 vaccines.
Please note: The Bump and the materials and information it contains are not intended to, and do not constitute, medical or other health advice or diagnosis and should not be used as such. You should always consult with a qualified physician or health professional about your specific circumstances.
Navigate forward to interact with the calendar and select a date. Press the question mark key to get the keyboard shortcuts for changing dates.